
Updated 26 November 2014

|
Updated 26 November 2014 |
|
HOME PAGES GLOBAL WARMING WHAT YOU CAN DO GREEN ISSUES BACKGROUND THE INITIATIVE |
Is Hydrogen the Fuel of the Future? Hydrogen can supply energy without significant effects on the planet. It can either be burned as a fuel or used to generate electricity in fuel cells. But the hydrogen must be produced from renewable resources, not fossil fuel, without generating greenhouse gases or pollution. To achieve these goals, serious cost, safety and technical problems must be overcome. This page covers the generation of hydrogen, its storage and transport, its use for fuel cells, examples of projects currently underway, and the general issues of its economics, safety and environmental problems. It ends a brief summary of the outlook. Hydrogen would appear to be the ideal fuel. It is available in great abundance and is non-toxic. The residue from burning pure hydrogen is simply water, without other pollution. It could be used as a fuel for power stations, for heating, and for use in land vehicles, aeroplanes and ships. It has already been used to power space rockets, and in prototype buses and cars. 
The advantages of using hydrogen to replace oil, gas and coal have been recognised for several decades, and a great deal of research and development has been devoted to this. However, the hoped-for hydrogen economy has been slow in coming. Nevertheless, hydrogen remains a potentially attractive option for combining a long-term solution to global warming with the freedom to expand energy usage to meet human demands. Much of the publicity gives the impression that hydrogen can simply be used as a clean energy source to replace fossil fuels, as soon as some challenging technical problems are solved. But this misses an important point. Unlike fossil fuels, which already exist, hydrogen has to be produced, and that uses energy. In effect, hydrogen acts as an energy storage medium rather than an energy source. So in deciding how useful hydrogen is we must take account of the way it is produced, as well as how it is distributed and used. At present almost all hydrogen is produced from fossil fuel. The ideal is to produce it from water, and to do so using renewable resources such as hydropower, wind or solar energy. It can also be produced by nuclear fission power, and possibly in the distant future by nuclear fusion. Hydrogen could be used to store energy from sources which don't work all the time, such as solar photovoltaic panels, or which generate energy when it is not needed, for example from nuclear power stations which are more efficient if run continuously. Hydrogen could also be used as a means for transporting energy. For example, solar energy installations in desert areas could extract hydrogen from water. The hydrogen would then be transported by pipeline or ship to where it is needed; this is discussed further below. In many cases, an alternative to hydrogen is the use of rechargeable batteries as an energy storage medium. It seems unlikely that the use of batteries will satisfy some applications – for example powering aeroplanes. However, the relative future importance and balance of battery solutions versus hydrogen solutions is difficult to predict, and depends on how the technologies develop. It may also prove more economic to create other fuels rather than hydrogen. On this page we will outline the situation, status and outlook. More detailed discussions on the hydrogen economy can be found in the Wikipedia and a report to the US Department of Energy. 
Hydrogen is a very energetic fuel by weight, yielding around 33 kilowatt-hours per kilogram. This is two and a half to three times the energy of the same weight of conventional fuels, such as natural gas or petrol. However, hydrogen has a very low molecular weight, and as a result it is a very light gas – one kilogram at atmospheric pressure occupies 11,200 litres. Liquid hydrogen is course denser, but petrol at room temperature is roughly 10 times denser still, so by volume liquid hydrogen actually has only about one-quarter of the energy density of petrol. This means that a basic problem with hydrogen is storage. If stored as a gas it needs to be highly compressed, and energy is needed to do that. And as a liquid hydrogen must be kept very cold since its boiling point is –252.8°C, or 20 K, and even more energy is needed to liquefy it and keep it cold. Hydrogen burns with a light blue flame which is not easy to see. To avoid people touching the flame accidentally, it is desirable to mix the hydrogen with a small proportion of a fuel such as methane to colour the flame. Burning hydrogen in air is not entirely benign, as some nitrous oxide (a potent greenhouse gas) is also produced. More serious is the problem of explosion. A wide range of hydrogen concentrations in air can explode if ignited, so care must be taken. Fuel cells (discussed below) allow the direct conversion of hydrogen energy into electricity. This is much more efficient than simply burning the hydrogen like a conventional fuel, for example in an internal combustion engine. 
Hydrogen is currently used in large quantities as an industrial chemical, to make ammonia for fertiliser and in processing crude oil to make fuel. Almost all of this hydrogen is produced from natural gas, oil and coal, as in the photo. Producing hydrogen from natural gas is fairly efficient, partly because waste heat from power stations is used in the process. However, the fossil-fuel sources are not renewable and carbon dioxide is also produced as a by-product, so this is not an acceptable solution. One emerging way around this is to use biogas, i.e. methane produced from food waste. Electrolysis of water, currently used to produce only about 4% of industrial hydrogen, could be far cleaner and more sustainable. Low temperature electrolysis 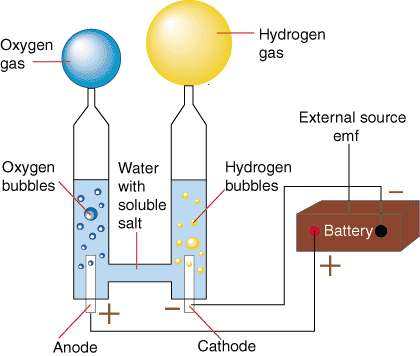
Electric current is passed between two electrodes immersed in water. Oxygen appears at the positive electrode and hydrogen at the negative electrode. The diagram illustrates the basic principle. The water must be pure as impurities can degrade the electrodes. The advantages of this process are that:
About 80% of the electrical energy can be stored as hydrogen. The remaining energy appears as heat, which must be removed. High temperature electrolysis If electrolysis takes place at a higher temperature, or even on steam, more of the energy used to create the hydrogen can be thermal energy, which is less expensive than electrical energy. However, the need for high temperatures rules out solar photovoltaic and wind energy. Heating by solar concentrators or by nuclear power would be possible. A 100-kilowatt pilot project in Spain, which concentrates sunlight to obtain the 800 to 1200 °C required to split water, is Hydrosol-2, which started up in 2008. In the case of nuclear power (which is opposed by many people), electrolysing water to make hydrogen could help in utilising the inflexible always-on operation at times of low electricity demand. Thermochemical production Thermochemical processes have been demonstrated which produce hydrogen and oxygen from water and heat, without using electricity. These could be more efficient than processes involving electrolysis, since the input energy is only heat. However, none has been implemented on a commercial scale. Very high temperature separation At temperatures of 3000 °C water molecules separate into their constituent hydrogen and oxygen. The necessary temperature can be reached by furnaces heated by solar concentrators, and the gases can then be separated by diffusion through a suitable membrane. This method of separation is currently only experimental; there are no commercial installations. * The energy for making hydrogen should ideally come from a cheap, clean, renewable source – we are still a long way from this goal. If hydrogen is stored at atmospheric pressure, its low density means that there is only about one-third as much energy per cubic metre as in natural gas. A gasometer holding the same energy reserves needs to be three times the size if hydrogen is the fuel. To reduce the storage volume needed, hydrogen must be stored in a compressed or liquefied state, or in some sort of chemical form. Hydrogen at 50 atmospheres pressure provides about 0.13 kilowatt-hours per litre, and liquid hydrogen provides 2.36 kilowatt-hours per litre. (Petrol or oil provide about 10 kilowatt-hours per litre.) As a gas At present, temporary storage of bulk hydrogen for later use is most cheaply done in suitable underground caverns. ICI stores hydrogen at 50 atmospheres pressure in old salt mines at Teesside. Stationary storage above ground uses similar pressures. If hydrogen is to be used as a transport fuel it must provide a sufficient driving range. It takes energy to compress the gas, and although compressing the gas improves the energy density, smaller tanks are not necessarily lighter than big ones because they must be stronger, and heavy tanks are not good for fuel economy. For example, a 100 litre tank of hydrogen at 250 atmospheres pressure holds about 2.2 kg of hydrogen providing 72 kilowatt-hours of energy. Such a tank might weigh 30 kg, far more than the hydrogen itself. By comparison, only 8.2 litres of petrol is needed to provide 72 kilowatt-hours of energy – this weighs about 6 kg plus a tank weighing a fraction of a kilogram. These figures also indicate that such a hydrogen tank would not provide very much driving range – well under 100 miles in today's most efficient cars. To match the driving range of a typical petrol car in this way would require a hydrogen tank of about 1 cubic metre. Hydrogen is a small, energetic molecule, so it tends to diffuse through any material intended to contain it. This causes embrittlement, or weakening, of tanks and pipes. As a liquid 
Liquid hydrogen presents more problems. An energy-intensive liquefaction process is needed to get to the required –252.8 °C, and then cryogenic storage must be used. Liquid hydrogen storage tanks must be well insulated to minimise boil-off. Due largely to the use of liquid hydrogen fuel for space rockets, good low-mass liquid hydrogen tanks have been developed. However, they will still be heavier and bulkier than petrol tanks, and for the same energy yield the hydrogen volume must be more than four times greater. The photo shows a liquid hydrogen fuel tank in the boot of a car. As a solid Instead of storing pure hydrogen, it can also be stored as a chemical hydride or some other hydrogen-containing compound. Hydrogen gas is reacted with other materials to produce the hydrogen storage material, which can be transported relatively easily. At the point of use the hydrogen storage material can be made to decompose, yielding hydrogen gas. Charging the metal hydride store with hydrogen generates heat, and heat must also be applied to the container to release the hydrogen. Barriers to practical storage schemes include the high pressure and temperature conditions needed first to form the hydride and then to release the hydrogen, which only comes out slowly. In addition, the hydrides either degrade very quickly when exposed to the oxygen in air or are very flammable. Interesting research at the Rutherford Appleton Laboratory in Oxfordshire, UK, is developing nanobeads that can be poured and pumped like a liquid. The hydrides attach to the beads, which are safer, have a longer life, and release the hydrogen much faster when heated. Compared with compressed hydrogen, storage as a hydride provides a higher energy density: 72 kilowatt-hours of hydrogen would need about a 20 litre lightweight container, and the hydride would weigh about 110 kg. But compared with the equivalent petrol tank, the hydride store is about three times the size and twenty times as heavy. Another approach is to absorb molecular hydrogen into a solid storage material. Unlike hydrides, the hydrogen does not combine chemically in the storage system, and hence does not suffer from some of the limitations of hydride storage systems. Hydrogen densities similar to liquefied hydrogen can be achieved with appropriate absorption media. However, the most common method of on-board hydrogen storage in today's demonstration vehicles is still the simple option of compressed gas, at pressures of up to 700 atmospheres. * The low density of hydrogen presents major unsolved problems for storage. The low density of hydrogen also means that pipeline distribution of compressed hydrogen gas is less efficient than a similar natural gas pipeline. As mentioned above, to get the same energy as natural gas three times the volume of hydrogen must be delivered, and this uses more energy. In addition, hydrogen accelerates the cracking of steel (embrittlement), which increases maintenance costs, leakage rates, and material costs. Well-established pipeline installations in the USA and Germany do exist to transport hydrogen over distances of up to 40 miles. However, hydrogen pipelines are more expensive than even long-distance electricity lines. 
Pressurised containers can also be used to transport gaseous hydrogen, at pressures up to 250 atmospheres, on lorries, trains, and ships. Liquid hydrogen can be transported in standard shipping containers which include the necessary cooling and insulation. Each container holds about 40 cubic metres of liquid hydrogen. Large-scale world-wide distribution of liquid hydrogen would require ships equipped with cryogenic tanks and cooling facilities. Hydrogen can also be stored for transport in the form of metal hydride, as described above, balancing convenience with a big weight and energy penalty. The problems of transport raise the important question of whether it is better to:
* Either way, the infrastructure for obtaining hydrogen where it is needed does not yet exist. Combustion vs. fuel cells The low-tech option of simply burning hydrogen in place of fossil fuels could be used for a variety of applications:
Transport is probably the main area of interest for hydrogen. However, fuel cells driving electric motors can convert much more of the energy of the hydrogen into motive power than an internal combustion engine. * Fuel cells are generally seen as a more appropriate technology than simply burning hydrogen. Another area where fuel cells might become widely used, if they are more efficient and cost-effective than batteries or portable generators, is to supply electricity in places where there is no mains electricity supply. Fuel cell basics 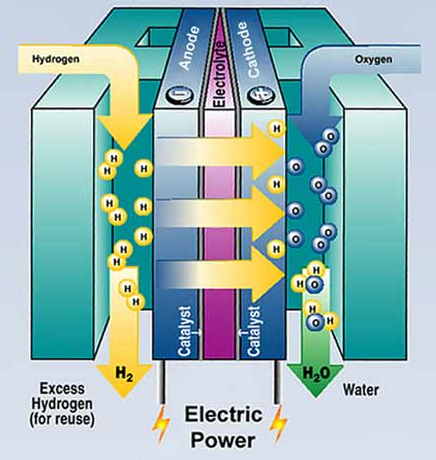
Fuel cells provide a very efficient way of converting the energy in the hydrogen into electricity. They were first made in about 1842 by a Welsh scientist, Sir William Robert Grove, and were first used in spacecraft in the 1960s. Since 1990 there has been intensive development for commercial applications. A fuel cell is an electrochemical conversion device. It produces electricity from fuel and an oxidant, which react in the presence of an electrolyte. The fuel and oxidant flow into the cell and the reaction products flow out of it, while the electrolyte remains inside. Fuel cells can operate virtually continuously as long as the necessary flows are maintained. The difference between fuel cells and batteries is that fuel cells must have external supplies of fuel and reactant, e.g. hydrogen and oxygen (usually from air), whereas batteries internally store electrical energy chemically. Other combinations of fuel and oxidant are possible: fuels include hydrocarbons and alcohols, and oxidants include chlorine and chlorine dioxide. In a hydrogen fuel cell the electrons and protons making up hydrogen are separated by a catalyst, typically platinum or a similar metal or alloy. The hydrogen's protons pass through an electrolyte. Its electrons, however, are forced to travel through an external circuit, thus producing electrical power. Another catalytic process takes the electrons back in, combining them with the protons and the oxidant to form water, the waste product. The diagram illustrates this. Other fuels than hydrogen produce carbon dioxide as well as water in the waste. In normal use a number of fuel cells are stacked in order to generate more electrical power. Types of fuel cells There are quite a few different kinds of fuel cells, with different applications. Alkaline Fuel Cells were the original type used on spacecraft. They are simple and cheap to make, but must be fed with pure hydrogen and oxygen since carbon dioxide degrades the electrolyte. 
Proton Exchange Membrane Fuel Cells (PEMFC) are very light, very efficient, and require only atmospheric oxygen instead of pure oxygen. A membrane allows the protons through and into the electrolyte, but forces the electrons to flow through the external circuit before they can combine with oxygen to form water. However, these fuel cells are very sensitive to carbon monoxide. The photo shows a stationary stack of PEMFCs with a rated output of 5 kilowatts. This is the favoured type of fuel cell at present for small-scale and portable applications, but the cost is still very high and needs to be reduced. Phosphoric Acid Fuel Cells (PAFC) are already in use for commercial combined heat and power applications. They are specific to large installations, as the cell becomes unusable if its temperature ever drops below 42 °C. Fuel cells with an electric power of 200 kilowatts and a thermal power of 220 kilowatts are available. Molten Carbonate Fuel Cells (MCFC) operate at around 600 °C. They can accept a variety of fuels including natural gas, and are not harmed by impurities. There is a fuller discussion, including many other types of fuel cells, in the Wikipedia. Examples of pilot projects and use for transport Iceland 
Due to abundant and cheap hydroelectric and geothermal energy sources, virtually the only use of fossil fuels in Iceland is for vehicles and fishing boats. (Photo shows a geothermal power station.) Therefore, it is not surprising that Iceland's plans for using hydrogen were advanced, especially as they can use hydrogen generated cleanly by geothermal energy. In 1998 Iceland committed to becoming the world's first hydrogen economy by the mid-21st century. In 2003 the world's first hydrogen station, like a petrol station, opened and three buses using fuel cells fed by compressed hydrogen started running in Reykjavík. These buses were part of a larger programme in a number of cities including London – see buses below. Every city bus in Reykjavík was supposed to run on hydrogen within less than a decade, followed by a switch to hydrogen-driven cars. Fuel cell-powered ships were being developed as well, with the aim of converting Iceland's 2500 fishing boats to hydrogen by 2015. However, this ambitious programme appears to have collapsed. The three buses in Reykjavík were decommisioned in 2007, and the only achievement of the ship programme was one tourist boat with a hydrogen-based auxiliary power system. Small islands Pilot projects demonstrating a hydrogen economy are under way or starting up on a number of small, isolated islands. For example, on the Norwegian island of Utsira the installation combines wind power and hydrogen power. In periods when there is surplus wind energy, the excess is used for generating hydrogen by electrolysis. The hydrogen is stored, and is available for power generation in periods when there is little wind. However, converting electricity to hydrogen and then back to electricity has proved to be very inefficient, and the low energy-density of hydrogen requires very large storage tanks. 
Hydrogen-powered buses are particularly advantageous in improving the air quality in cities, since their exhaust is just water vapour – much cleaner than diesel buses which emit polluting particulates and oxides of sulphur and nitrogen. Three Mercedes Citaro buses powered by hydrogen fuel cells operated in London for three years, starting in 2004. This was part of a European programme called Clean Urban Transport for Europe (CUTE); Madrid, Barcelona, Berlin, Hamburg, Amsterdam, Luxembourg, Perth, Reykjavik and Beijing also participated. The aim was to test first-generation fuel cell-powered buses. Eight hydrogen buses of an improved design were put into service in London starting in December 2010, though three of the eight were delivered late. These buses use their hydrogen fuel cells to recharge batteries that drive electric motors. Energy generated during braking is also used for recharging. This hybrid system makes them more efficient than the buses used in the CUTE pilot study, and they can run for up to 18 hours before they need to refuel. If the buses prove to be reliable then more may be ordered. However, they cost much more – £1 million each – than the diesel-electric hybrids now coming into routine use in many cities and towns. How many more hydrogen buses might be introduced in London is uncertain, depending on whether costs can be reduced. There are now about five hydrogen refuelling stations in and around London. Cars and other vehicles Many of the main car manufacturers have produced a wide variety of hydrogen-fuelled cars. A list can be found here. However, most of these are prototypes or one-off demonstrations. And in 2009 the US stopped supporting developments that encouraged hydrogen cars and instead supported battery-powered electric cars. Some, such as the BMW Hydrogen 7 (100 produced) use internal combustion engines – the BMW can burn petrol as well as hydrogen (which is stored in liquid form). Others, such as the Mercedes F-cell, use fuel cells fed by compressed hydrogen to drive electric motors. Some of the concept cars have also been hydrogen-driven electric/petrol hybrids. However, the main interest is in fuel cells, as they are much more efficient and thus allow the cars to go have much better ranges than cars burning hydrogen or current electric models powered by batteries. 
The first hydrogen car to go into small-scale production beyond the level of a few prototypes was the Honda FCX Clarity, shown at left. It is powered by a 100 kilowatt hydrogen fuel-cell stack. Electricity is stored in a lithium-ion battery, to give higher output when needed. Its range of 240 miles is provided by 3.9 kg of hydrogen stored at 150 atmospheres pressure. Limited leasing of about 200 cars began in June 2008 in California. However, there are only nine hydrogen filling stations, limited to the Los Angeles area,and this model did not move to mass production. An improved design, the Honda FCV Concept, is due to go on sale in Japan in 2016. 
Toyota plans to start marketing hydrogen cars in the hope that they will repeat the success and of its hybrid models, which pioneered hybrid technology and have led the market. The Toyota Mirai (shown at right) will be on sale in Japan, the US and Europe in 2015. Initially only 700 will be be sold, of which only 50–100 are expected to be shipped to the UK, Germany and Denmark in Europe. Although expensive (about £52,000 in Europe), even that price is less than the actual production cost. The range is claimed to be 300 miles, and the hydrogen tank that feeds the fuel cell can be refilled in a few minutes – assuming a filling station is within range. Toyota plans to work with Air Liquide to build a network of filling stations in the north-eastern US before starting to sell the car there starting in 2016. California plans to build more filling stations as well. For a number of years, Hyundai has had prototype hydrogen versions of the
Hyundai
Tucson/ix35 SUV,
and recently announced
that it will begin limited mass-production of the current version of this model
in 2015. It has a range of 350 miles. Hyundai is working with Transport for
London, ITM Power, Air Products and Johnson Matthey to develop more hydrogen
filling stations in the
UK, including outside London. In 2010 a prototype London taxi, powered by hydrogen fuel cells charging lithium-ion batteries and built by Lotus, was shown. A fleet of five taxis was in operation by 2012 and there are vague plans to expand this, but details are difficult to find. Hydrogen technology is too new, and too little used at present, to be directly competitive with technologies which have many years of experience and a mass market to support them. Thus its first practical applications would likely be cases where current technologies do not provide a suitable solution. Prime examples might be:
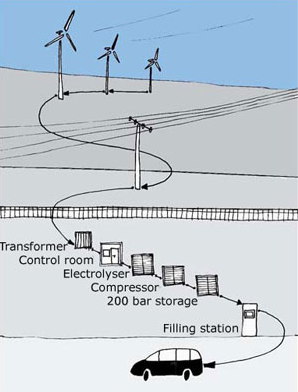
Currently, fuel cells are much more expensive to produce than internal combustion engines but are becoming cheaper as new technologies and production systems develop. Fuel cells can use other materials as their energy source, but the vision is to use hydrogen. If the cost of fuel cells could be reduced to the point where some important applications can use them effectively, mass production would then drive the cost down further as new techniques are developed. For use in cars, there are possible attractions beyond clean emissions. Fuel cells driving electric motors are lighter in weight and much more efficient than conventional engines. However, as noted already, hydrogen storage is a major problem, and so is the cost of making the fuel cells. At present it also takes more than twice as much energy to make a hydrogen fuel cell than it produces during its lifetime. Fossil fuels such as oil and gas are often used as the cost reference, but this is not a fair comparison. No allowance is made for the millions of years it took to make these fuels in the earth, yet the energy used for hydrogen production does get counted, even if it comes from a renewable source such as solar energy. It is better to compare hydrogen with other systems using renewable energy. One company claiming progress in reducing the cost and efficiency of proton exchange membrane fuel cells is ITM Power, which is developing fuel cell systems using hydrogen produced by off-grid renewable resources. They have developed much cheaper, platinum-free membranes. Converting to a hydrogen economy would require a lot of new infrastructure for distribution and storage, though what would be needed depends on how and where the hydrogen is generated and used. On the other hand, electric vehicles using batteries can use the existing electricity grid or local renewable generation for recharging, and much of this could be done at night when the grid is not heavily used. * Hydrogen must be shown to be more efficient, practical and cost effective than other renewable technologies, most notably rechargeable batteries. A confusion in introducing the hydrogen economy is the popular linkage of the term with the hydrogen bomb. There are, of course, no radioactivity problems in the uses of hydrogen being proposed here. A totally different use of hydrogen, nuclear fusion, is discussed on another page. 
More realistic concerns include the following:
Possible Environmental Problems Carbon dioxide produced during production of hydrogen If hydrogen is produced from directly from fossil fuels, or by electrolysis of water using fossil-fuel generated electricity, carbon dioxide will be produced that will add to global warming unless it is captured and stored. The obvious solution is to use electricity generated by intermittent renewable sources, and regard the hydrogen as a storage medium to use when the renewables are not available. Ozone-layer depletion Escaped hydrogen gas in the stratosphere might form free radicals due to ultraviolet radiation. These could act as catalysts for ozone depletion .The amount of leakage from even widespread use of hydrogen is probably too little to be a problem, but this underlines the need to minimise leakage, which is necessary anyway on safety grounds. Water is a greenhouse gas The unavoidable product of using hydrogen is water, so would water vapour from the exhausts of millions of vehicles add to global warming? In fact, the volume of water in the atmosphere created by burning hydrogen would be negligible compared with that generated by natural processes. The main concern might be the deposit of water at high altitudes by aeroplanes fuelled by hydrogen. Disposal of the fuel cells Fuel cells have a limited life, and there may be difficulties in recycling the materials from which they are made. Hydrogen is fairly likely to have some role in energy supplies in the future – the question is how big a role, and what it will be. Electricity generation – The main use of hydrogen might be to provide flexibility: hydrogen production when there is spare capacity, and generation using hydrogen when demand becomes very high and other renewable sources are not available. This limited role is because producing hydrogen using electricity, transporting and storing the hydrogen, and then generating electricity from the hydrogen cannot be very efficient. 
The use of solar power in distant tropical desert areas might provide another application. Very long transmission lines may prove to be much more expensive than producing and shipping hydrogen. This is discussed in more detail on our green energy page. Fuel cells could take over from batteries in providing back-up when mains power fails, or for off-grid use. Space heating – In the long term, better buildings can greatly reduce the consumption of energy for space heating. Hydrogen might have a role to play in providing clean heating, but there are other technologies that might prove to be more cost effective, such as heat pumps and wood burners. Fuel for industrial processes – Many processes, notably industrial ones (for example processing of metals), require high temperatures. A clean fuel source is needed, and hydrogen may well be the solution in place of such fuels as oil and gas. Transport – For several years the most likely application of hydrogen has looked to be transport, but few promises have been met. Although both direct combustion and fuel cells are being tried, fuel cells driving electric motors seem to be the better choice. But the alternative of using batteries, if possible recharged using mains electricity from renewable sources, is available now – albeit with limited range. At least for cars, buses and lorries (and possibly ships) it is not yet clear which will turn out to be more effective. For trains, renewable mains electricity seems to be the best solution. For aeroplanes batteries seem to be too heavy, so if hydrogen storage tanks can be made light enough then hydrogen might be a possible fuel source. |
 |
If you have any comments or queries about this website please contact the
webmaster. |
© Blewbury Energy Initiative 2014 |